Advances in the separation and analysis of AAV-based gene delivery vectors

Live30 webinars are thirty-minute presentations designed to update you on the latest innovations, applications, and data in a fast yet interactive format.
Recombinant AAVs are essential tools for gene delivery in treating genetic disorders. However, producing large quantities of high-quality AAV vectors with the target payload remains a significant challenge. Additionally, there is an increased demand for rapid, accurate, and robust analytical methods to assess critical quality attributes, such as the AAV empty-full ratio.
In this webinar, we present a novel Q-type anion exchange chromatography (AEX) method for efficiently separating and quantifying empty-full AAV capsids. This approach utilizes non-toxic choline-based mobile phase gradient salts and is applicable to several clinically relevant serotypes, including AAV2, AAV5, AAV6, and AAV8. We further characterize AAV vectors using size exclusion chromatography (SEC) coupled with UV absorption and multi-angle light scattering detectors to confirm empty-full ratios and identify impurities.
A significant potential application for choline-Cl in AEX chromatography is in AAV purification platforms with Q-type process resins. We further outline a method that selectively removes empty capsids from the full capsid pool using a choline chloride gradient, effectively enhancing the production process. This robust and scalable approach offers an environmentally friendly pathway for improving the production and analytical characterization of full capsids across multiple serotypes.
Attend this webinar to:
- Explore innovative AEX chromatography techniques for the processing and analysis of AAV viral vectors
- Discover the use of non-toxic, choline-based salt gradients for effective separation of empty and full AAV capsids
- Learn how a robust and scalable AAV purification method can be optimized for manufacturing and understand the broad applicability across various clinically relevant AAV serotypes
You might also like
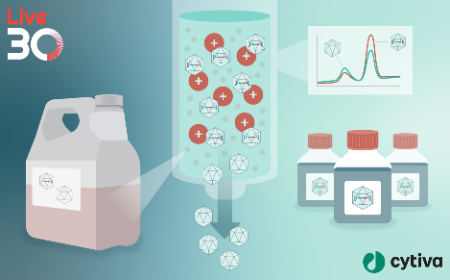
Achieving robust and scalable AAV empty/full capsid separation for gene therapy
Advancing cell therapy: innovations in polymer-based encapsulation and delivery
Increasing efficiency in AAV-based gene therapy production: platform optimization with multiserotype AAV affinity capture



