Filter by Interests
Filter by ContentType
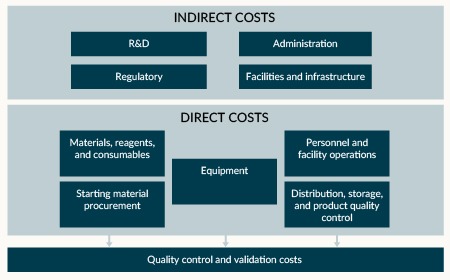
Choosing the best path to patients: how to decide between in-house and outsourced iPSC therapy development and manufacturing
Laura Koivusalo, Anni Mörö, Ross Macdonald
11 March 2025
Commentary
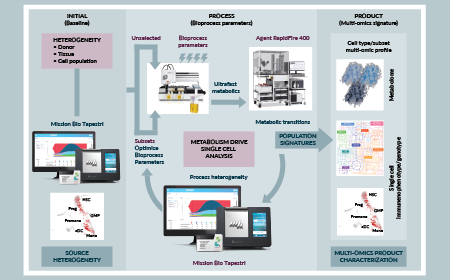
Personalization in advanced cellular ImmunoTherapeutics: activities in Ireland
Athanasios Mantalaris, Nicki Panoskaltsis, Fiona Killard
13 January 2025
Commentary
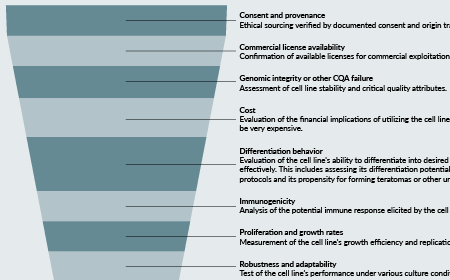
De-risking pluripotent stem cell therapies
Stephen Sullivan, Ricardo Baptista
18 June 2024
Commentary
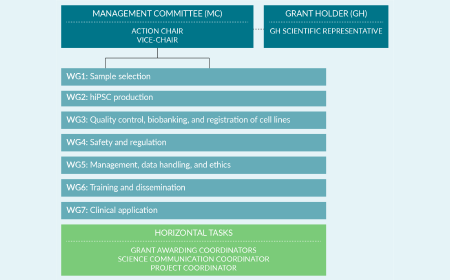
Unlocking the full potential of human induced pluripotent stem cells from haplo-selected cord blood samples—the how and the why
B Aran, D Morrow, E Rodriguez et al
14 February 2024
Commentary
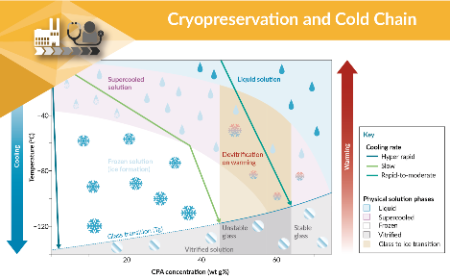
Innovation in cryopreservation & cold chain management
Barry Fuller, Roland Fleck, Glyn Stacey
22 November 2023
Commentary

Cell and gene therapy commercial off-the-shelf software components
Parris Farr
23 August 2023
Commentary
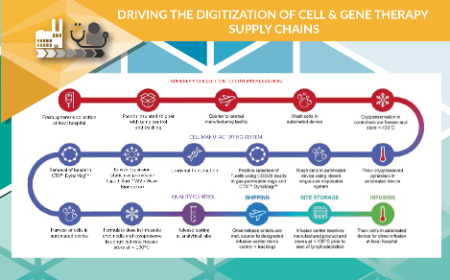
Critical success factors to consider before digitizing a cell & gene therapy supply chain
August Zepka, Charles Wilfong
23 July 2022
Commentary
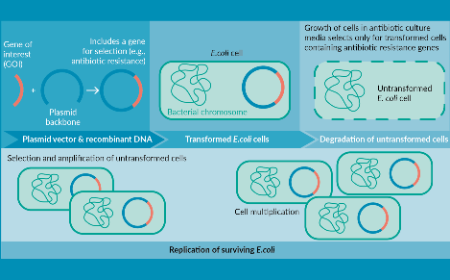
Regulatory & supply chain implications for plasmids as critical starting materials in the manufacture of viral vector gene therapy products
M Gosse, C Jones, D Jesus et al
08 March 2022
Commentary

Challenges in qualification & management of raw materials in advanced therapy medicinal products
Shok Ping Lim, Sakina Gooljar
04 March 2022
Commentary

Master ATMP processes by controlling raw and starting material
Anne-Sophie Lebrun, Carmen Brenner
24 March 2021
Commentary

Considerations for performing virtual quality audits on manufacturers of gene therapy viral vectors: an auditor’s perspective during the COVID-19 public health emergency
Gary C du Moulin, PhD, MPH, RAC
18 March 2021
Commentary
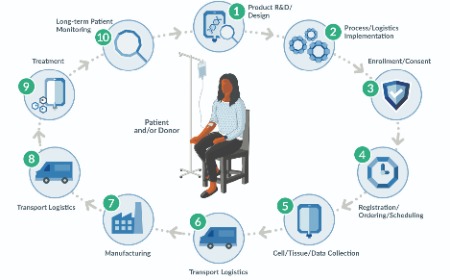
Small labels, big challenges: solutions for advanced therapy labeling
Heidi Hagen, Christophe Suchet
21 September 2020
Commentary

The role of raw materials in the manufacturing of cell and gene therapy medicinal products: practices and challenges
José Caraballo
09 April 2020
Commentary