Filter by Interests
Filter by ContentType
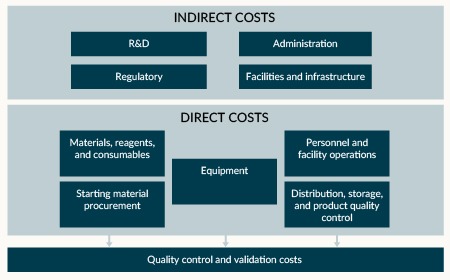
Choosing the best path to patients: how to decide between in-house and outsourced iPSC therapy development and manufacturing
Laura Koivusalo, Anni Mörö, Ross Macdonald
11 March 2025
Commentary
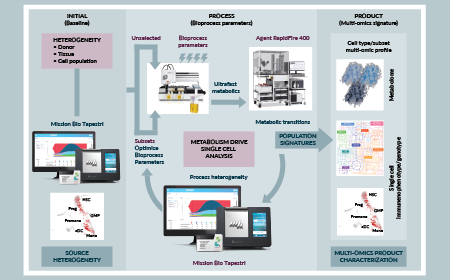
Personalization in advanced cellular ImmunoTherapeutics: activities in Ireland
Athanasios Mantalaris, Nicki Panoskaltsis, Fiona Killard
13 January 2025
Commentary
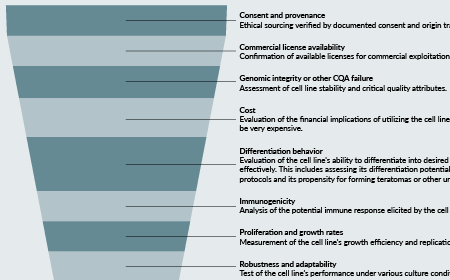
De-risking pluripotent stem cell therapies
Stephen Sullivan, Ricardo Baptista
18 June 2024
Commentary
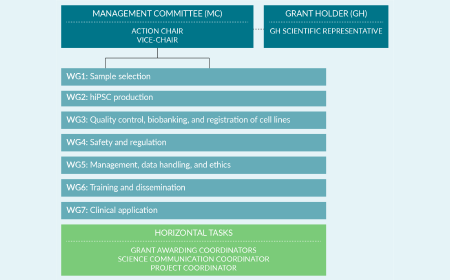
Unlocking the full potential of human induced pluripotent stem cells from haplo-selected cord blood samples—the how and the why
B Aran, D Morrow, E Rodriguez et al
14 February 2024
Commentary
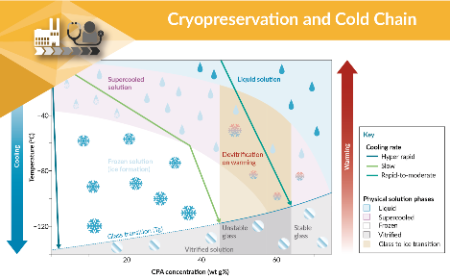
Innovation in cryopreservation & cold chain management
Barry Fuller, Roland Fleck, Glyn Stacey
22 November 2023
Commentary

Cell and gene therapy commercial off-the-shelf software components
Parris Farr
23 August 2023
Commentary
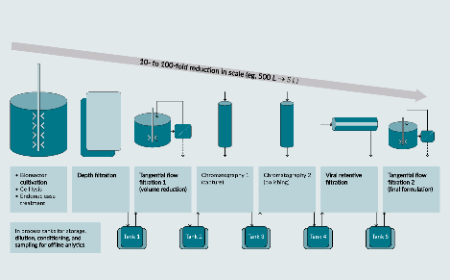
Opportunities to implement continuous processing in production of recombinant adeno-associated viral vectors
G Thakur, S Mink, H Bak et al
06 June 2023
Commentary
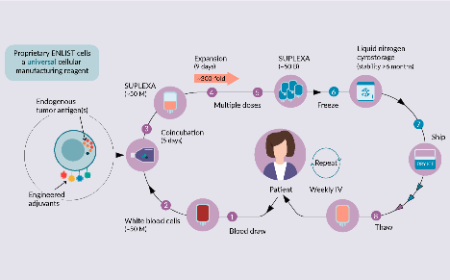
Alloplex Biotherapeutic’s SUPLEXA cells represent a new type of autologous adoptive cellular therapy for cancer
Frank Borriello, James A Lederer
25 April 2023
Commentary
Overcoming challenges to CAR-T cell therapies in India
A Uppal, R Chakrabarti, N Chirmule et al
08 March 2023
Commentary
Cell therapy 101 (part 1)Challenges of early clinical development: a ‘thumbnail sketch’
Michael D Leek
29 November 2022
Commentary
Why we need a revolution for personalized cell therapies
S Steiner, B Gilgen, E Pernot et al
06 July 2022
Commentary
COVID-19: how is the pandemic changing the vaccines space?
Nicholas Jackson
24 May 2022
Commentary
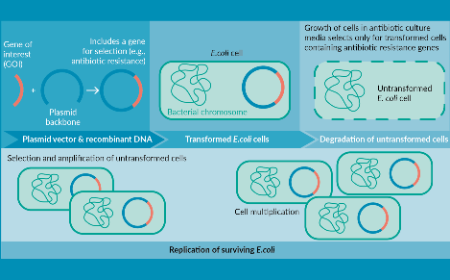
Regulatory & supply chain implications for plasmids as critical starting materials in the manufacture of viral vector gene therapy products
M Gosse, C Jones, D Jesus et al
08 March 2022
Commentary

Challenges in qualification & management of raw materials in advanced therapy medicinal products
Shok Ping Lim, Sakina Gooljar
04 March 2022
Commentary
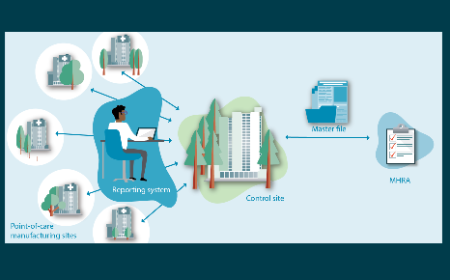
The UK’s emerging regulatory framework for point-of-care manufacture: insights from a workshop on advanced therapies
E Bicudo, I Brass, P Carmichael et al
30 September 2021
Commentary
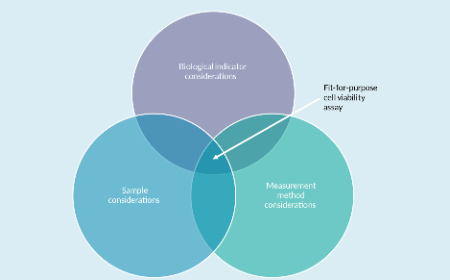
Outcomes from a cell viability workshop: fit-for-purpose considerations for cell viability measurements for cellular therapeutic products
L Pierce, S Sarkar, L Li-Ying Chan et al
14 May 2021
Commentary
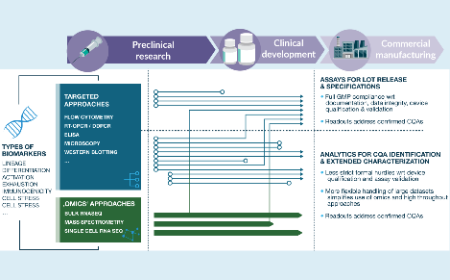
Finding quality in complexity: how cellular therapeutics are shifting analytical paradigms for clinical supply and product manufacturing
Vilma Jimenez Sabinina, Dominic Günter Hildebrand
13 May 2021
Commentary
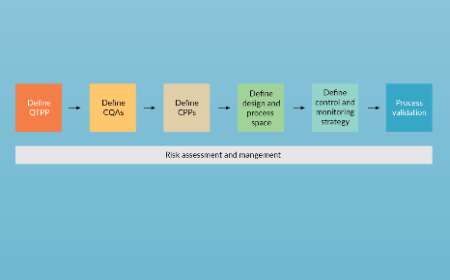
Making the move from antibody therapeutics to gene therapy: applicability of monoclonal antibody learnings to adeno-associated virus vector bioprocessing
Andrew D Tustian
29 April 2021
Commentary

Yesterday, today and tomorrow: the evolving landscape of gene therapy manufacturing and process development
David R Knop, PhD
29 April 2021
Commentary

Future-proofing cell therapy manufacturing capability: lessons from the NCI
Anthony Welch, Marc Ernstoff, Jason Yovandich
20 April 2021
Commentary

Master ATMP processes by controlling raw and starting material
Anne-Sophie Lebrun, Carmen Brenner
24 March 2021
Commentary