Filter by ContentType

‘Winning’ target product profiles for CAR-T cell therapies in oncology: critical success factors for commercially viable therapies
Clare Hague, Louise Street-Docherty, Frances Pearson
19 December 2024
Expert Insight
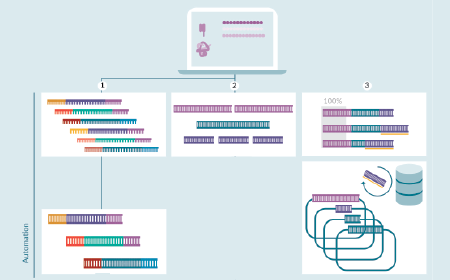
Automated plasmid assembly: a perspective from early drug discovery
S Ahammu, J Chi, T Edström et al
18 December 2024
Expert Insight
Interpreting the new FDA draft potency guidance: an RNA cell therapy perspective
Damian Marshall, Kayleigh Thirlwell
02 December 2024
Expert Insight
Operations strategy for a scalable CGT supply chain: key CXO insights
Shesh Sharma, Tim Sirichoke, Edward Ballesteros
02 December 2024
Expert Insight

Revolutionizing the treatment of cancer with allogeneic CAR-T cell therapy
Cokey Nguyen
13 November 2024
Expert Insight
Enhancing AAV process quality and efficiency: three case studies highlighting the benefits of upgraded analytics on downstream process development
E Hudspeth, I Green, T Dewosky et al
11 November 2024
Expert Insight
Challenges encountered when delivering advanced therapy investigational medicinal products in the National Health Service in the UK: a pharmacy perspective
B Amirloo, B Duran, I Sanna et al
29 October 2024
Expert Insight
Analytics for cell and gene therapy products in early development: points to consider before preparing an IND for a first-in-human clinical trial
William E Janssen, Scott Burger
15 October 2024
Expert Insight
Therapeutic epigenome editing: safety and quality considerations of a new class of gene-targeted medicines
Houria Bachtarzi, Tim Farries
15 October 2024
Expert Insight
The cell therapy industrial revolution: current successes, existing challenges, and expansion into new domains
Joel Gaston, Matthew Li
24 September 2024
Expert Insight
Patient-specific factors that could influence gene therapy outcomes in sickle cell disease
Abdulrahman Alsultan, Jaap Jan Boelens
02 September 2024
Expert Insight

Upstream processing of viral vectors: a summary
Pardhasaradhi Mathi
29 July 2024
Expert Insight

MHRA regulatory considerations for the quality of mRNA products
Ka-Wai Wan, Francis Galaway
22 May 2024
Expert Insight
The sweet cell of success: key considerations for the sourcing and production of pluripotent cell lines for therapeutic development
Samuel JI Blackford, Nathan C Manley
21 May 2024
Expert Insight
Raw materials and supplies for cell therapies: end to end expectations and best practices
Lili Belcastro
15 May 2024
Expert Insight
Are we there yet? After 250+ AAV-based clinical trials, do we have a well-paved road toward first-in-human entry?
Long Chen, Chia Chu, Francesca Vitelli
10 May 2024
Expert Insight
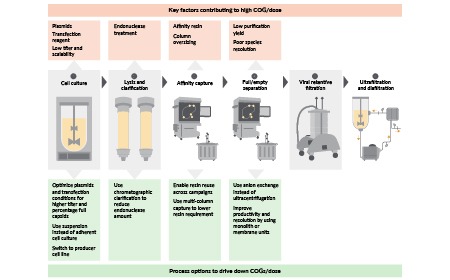
Improving process efficiency to reduce cost-of-goods per dose in manufacturing of recombinant AAVs
G Thakur, S Mink, H Bak et al
03 May 2024
Expert Insight
Innovation in hematopoietic stem cell cryopreservation and cold chain management
M Prisciandaro, M Santodirocco, G Fania et al
30 April 2024
Expert Insight
Preclinical trends in developing genetically altered cell therapies
Mary Ellen Cosenza
25 March 2024
Expert Insight
Challenges and advances of the stability of mRNA delivery therapeutics
Jin Zhai, Trystin Cote, Yupeng Chen
13 March 2024
Expert Insight

A straightforward tool for developing a raw material supply strategy for cell & gene therapies
K Kulenkampff, R Eigenmann, D Karlstetter et al
08 January 2024
Expert Insight