Filter by Interests
Filter by ContentType
Automated plasmid assembly: a perspective from early drug discovery
S Ahammu, J Chi, T Edström et al
18 December 2024
Expert Insight
Operations strategy for a scalable CGT supply chain: key CXO insights
Shesh Sharma, Tim Sirichoke, Edward Ballesteros
02 December 2024
Expert Insight

Revolutionizing the treatment of cancer with allogeneic CAR-T cell therapy
Cokey Nguyen
13 November 2024
Expert Insight
Enhancing AAV process quality and efficiency: three case studies highlighting the benefits of upgraded analytics on downstream process development
E Hudspeth, I Green, T Dewosky et al
11 November 2024
Expert Insight
Analytics for cell and gene therapy products in early development: points to consider before preparing an IND for a first-in-human clinical trial
William E Janssen, Scott Burger
15 October 2024
Expert Insight
The cell therapy industrial revolution: current successes, existing challenges, and expansion into new domains
Joel Gaston, Matthew Li
24 September 2024
Expert Insight

Upstream processing of viral vectors: a summary
Pardhasaradhi Mathi
29 July 2024
Expert Insight
The sweet cell of success: key considerations for the sourcing and production of pluripotent cell lines for therapeutic development
Samuel JI Blackford, Nathan C Manley
21 May 2024
Expert Insight
Raw materials and supplies for cell therapies: end to end expectations and best practices
Lili Belcastro
15 May 2024
Expert Insight

A straightforward tool for developing a raw material supply strategy for cell & gene therapies
K Kulenkampff, R Eigenmann, D Karlstetter et al
08 January 2024
Expert Insight
Considerations for development of gene-edited PSC-based therapies
Brent Morse, Amanda Mack
14 November 2023
Expert Insight
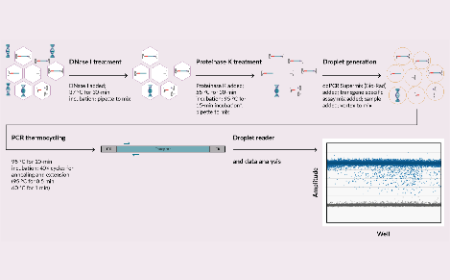
Optimizing ddPCR assay for characterizing AAV vector genome integrity
Aishwarya Shevade, John S Reeves, Andrew D Tustian
03 August 2023
Expert Insight
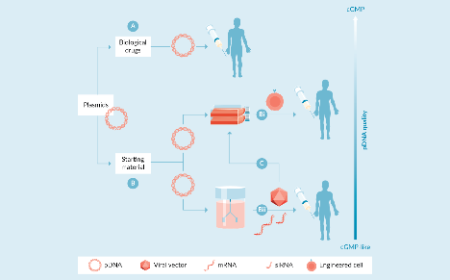
The supporting role of plasmids in gene & cell therapy
Duarte Prazeres
06 July 2023
Expert Insight
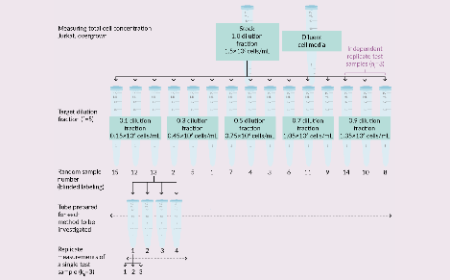
A guided demonstration of the Counting Method Evaluation Tool (COMET) for implementing the ISO 20391-2 Cell Counting standard
L Pierce, D Newton, S P Lund et al
21 June 2023
Expert Insight
Challenges in obtaining cellular therapy starting material for patients with sickle cell disease
Yvette C Tanhehco
22 March 2023
Expert Insight
Taking lessons from nature to improve cell therapy cryopreservation
Jason Acker, Nishaka William, Mackenzie Coatham
20 March 2023
Expert Insight
Exosomes: next generation vectors for drug delivery & gene therapy
Jess Morhayim, Jeroen de Vrij
12 December 2022
Expert Insight
Harnessing the power of design of experiments for cell therapy research & process development
Sean O’Farrell
30 November 2022
Expert Insight
Expanding the analytical toolbox to address complex cell-based therapies
Nathan C Manley, Samuel JI Blackford
18 November 2022
Expert Insight
Strategic partnering to enable cell therapy commercialization
02 October 2022
Expert Insight
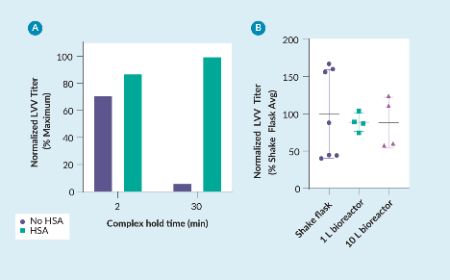
Stabilizing DNA–PEI complexes improves scalability of suspension lentiviral viral vector and AAV processes
B Olden, H Seo, R Barnes et al
28 August 2022
Expert Insight