As the cell and gene therapy industry continues to rapidly evolve, efficient, reproducible, scalable, and cost-effective viral vector manufacture is of critical importance. Adenovirus, retrovirus, lentivirus, and adeno-associated virus (AAV) remain the most commonly used vectors for the delivery of in vivo and ex vivo gene therapies, and a successful viral manufacturing pipeline must be able to deliver a consistent, pure, and high-titer product that exhibits good safety and efficacy.
At the same time, non-viral delivery platforms such as mRNA continue to evolve and grow in terms of their utility for the advanced therapies field, presenting novel challenges and opportunities for bioprocess developers.
For the viral vector field in particular, downstream processing requires the purification of virus particles from process-and product - related impurities including host cell material, plasmid DNA, and empty capsids. In addition, downstream processing of both viral vectors and mRNA can represent a significant portion of the total cost of production, so effective methods of generating high purity vector product are crucial.
The following collection of curated content from Thermo Fisher Scientific explores the key role that viral vectors and mRNA play in cell and gene therapy, the current challenges facing the field, and potential solutions for successful vector manufacture.
Any Questions?
Content
Process optimization of mRNA purification for vaccines and therapeutic applications

5 best practices for lentiviral purification and downstream processing success
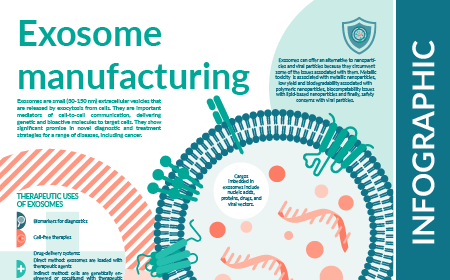
Exosome manufacturing: INFOGRAPHIC
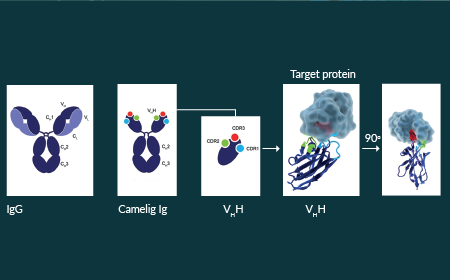
Advancing gene therapy development with a multi-serotype AAV affinity resin

Affinity chromatography for malaria vaccine purification

Streamlining vaccine development & production with modality-specific purification tools for mRNA & beyond

Trends and Innovation in Lentiviral Vector Processing

Advancing the purification of VSV-G pseudotyped lentiviral vectors by using affinity chromatography

Driving the expansion of mRNA into the therapeutic sphere

Enabling rapid vaccine development through manufacturing innovation & process efficiency
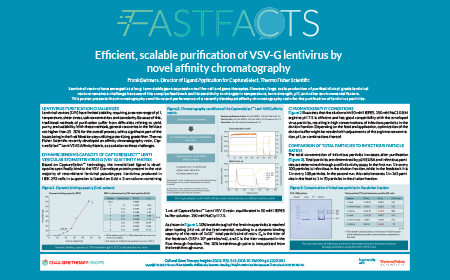
Efficient, scalable purification of VSV-G lentivirus by novel affinity chromatography

Advancing the purification of VSV-G pseudotyped lentiviral vectors by using affinity chromatography

Simplifying lentiviral downstream processing with a novel affinity resin & robust analytical tools
Advancing the purification of VSV-G pseudotyped lentiviral vectors by using affinity chromatography

Simplifying lentiviral downstream processing with a novel affinity resin & robust analytical tools

Driving the expansion of mRNA into the therapeutic sphere
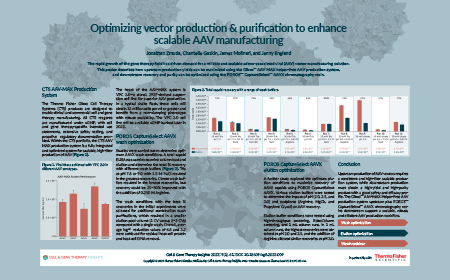
Optimizing vector production & purification to enhance scalable AAV manufacturing

Manufacturing considerations underpinning viral & non-viral platform selection

Optimizing vector production and purification to enhance scalable AAV manufacturing

Simplifying lentiviral downstream processing with a novel affinity resin and robust analytical tools
Addressing current challenges in lentiviral vector purification and associated analytics
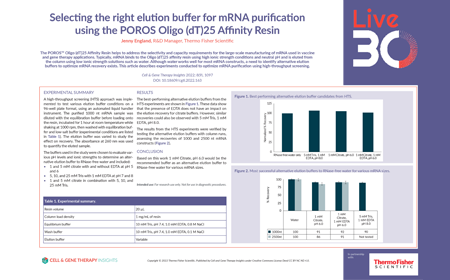
Selecting the right elution buffer for mRNA purification using the POROS Oligo (dT)25 Affinity Resin

POROSTM CaptureSelectTM AAVX elution optimization study for optimized recovery of AAV6 capsids

Optimizing vector production and purification to enhance scalable AAV manufacturing

Simplifying residual DNA analysis in viral vector production: focus on E1A

How can we maximize the efficiency of large-scale LV vector production?

Manufacturing considerations underpinning viral and non-viral platform selection
Mycoplasma testing regulatory guidance and strategies for cGMP cell and gene therapy manufacturing

Optimizing mRNA purification conditions by using a High Throughput Screening approach

POROS CaptureSelect AAVX Wash Optimization Study for Optimized Recovery and Purity of AAV6 Capsids
Manufacturing considerations for viral and non-viral platform selection

Ebook: Viral Vector Purification

Current technological trends & advancements in vector purification
Optimizing downstream purification of high-quality plasmid DNA with POROS™ Chromatography Resins
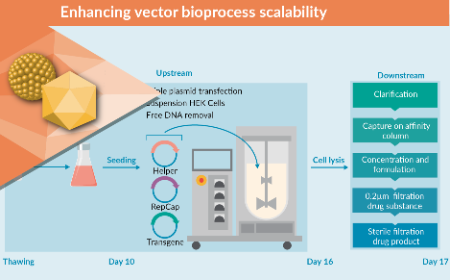
Platform optimization for efficient AAV purification: insights from a CDMO

Current technological trends and advancements in vector purification
Platform optimization for efficient AAV purification – insights from a CDMO

Expert Roundtable: evolution in AAV process development: 2022 and beyond

Optimizing downstream purification of high-quality plasmid DNA for gene therapy and vaccine production
Key lessons for cell & gene therapy and mRNA therapeutic development from COVID-19 mRNA vaccine approvals

Supporting development of mRNA-based therapies by addressing large scale purification challenges

Scalable AAV manufacturing: addressing challenges across the workflow
Development and validation of quantitative real-time PCR for the detection of residual HEK-293 host cell DNA update

Downstream purification of adeno-associated virus for large-scale manufacturing of gene therapies

Expert Roundtable: getting a gene therapy product to market: pitfalls and how to prevent them
AAV downstream processing for industrial scale production – moving towards commercial manufacturing
Development and validation of quantitative real-time PCR for the detection of residual HEK-293 host cell DNA
Accelerating advancement in gene therapy by improving downstream purification of viral vectors
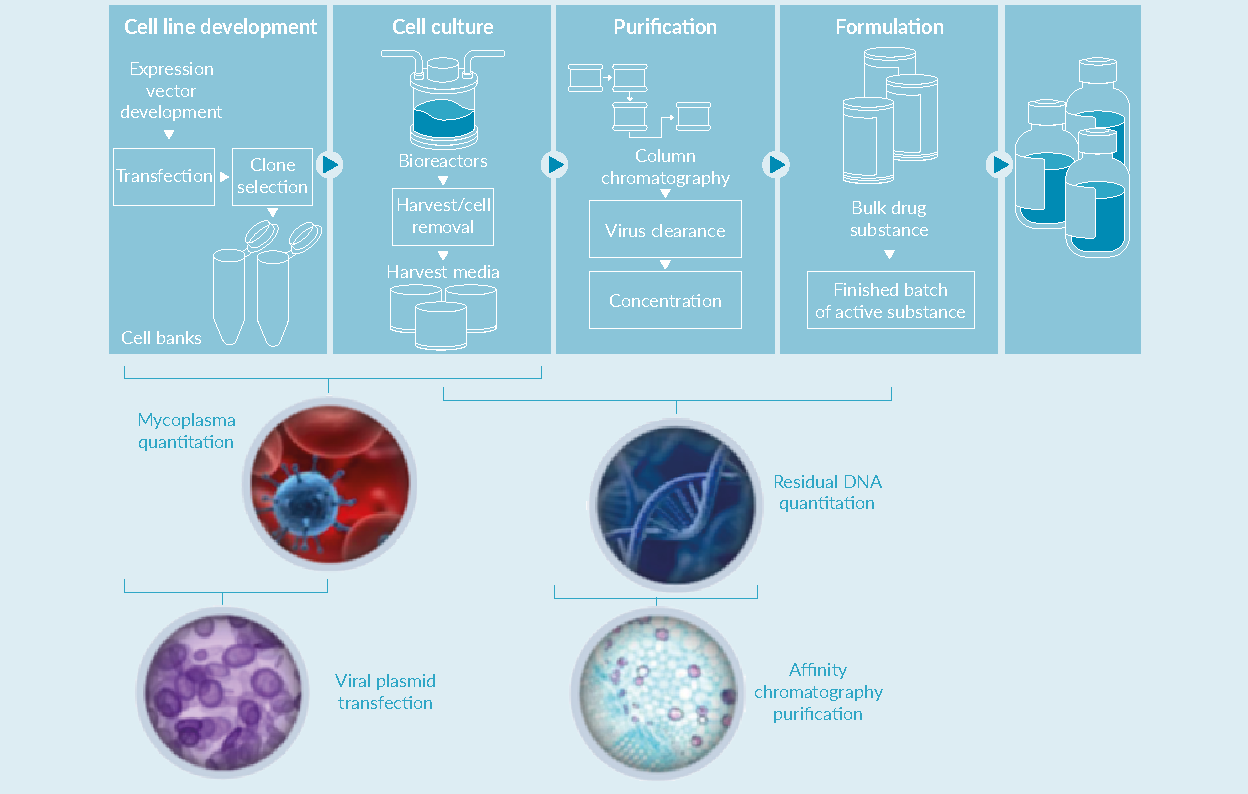
Upstream and downstream solutions for AAV manufacturing

Breakthrough Therapy Designation & Regenerative Medicine Advanced Therapy Designation Programs in Cellular & Gene Therapies