Filter by Interests
Filter by ContentType

Ensuring compliance through collaboration: managing raw material changes in cell and gene therapy regulatory filings
Kasey Kime, Xiao Peng
20 March 2025
Innovator Insight
Worth the switch? Enhancing process performance for cell therapy manufacturing with an animal component-free raw material strategy
Phil Morton, Shanya Jiang
03 March 2025
Innovator Insight
Next-generation risk mitigation in the temperature-controlled supply chain for advanced therapies with ISO 21973 compliance
Edward Grimley, Leanne Kodsmann
04 December 2024
Innovator Insight
Regulatory considerations & validation strategies for mycoplasma testing for cell-based therapies
Michael Brewer
14 September 2023
Innovator Insight

Addressing regulatory guidance for HEK293 cells & AAV-based therapeutics manufacturing
Michael Brewer
23 August 2023
Innovator Insight

Commercializing cell & gene therapies: a perspective from the quality function
Christoph Meyer, Andreas Wirth
09 February 2023
Innovator Insight

Automating the final cell therapy bioprocess step for robust CMC/GMP compliance
Tracy Moore, Delara Motlagh
07 February 2022
Innovator Insight
Understanding raw material performance: quality and consistency of cytokines for translation to the clinic
Bernd Leistler
18 October 2021
Innovator Insight

Navigating regulations: novel cell therapy platforms and their path to clinical manufacturing
N Bauer, N Calhoun, A Davies et al
11 August 2021
Innovator Insight
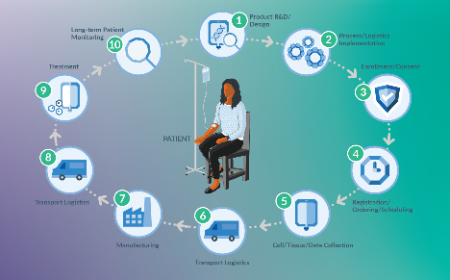
CMC obstacles in cell and gene therapy: four solutions to solve six challenges
Subbu Viswanathan, Marc Puich
10 May 2021
Innovator Insight
Developing an understanding of the analytical landscape for testing complex biological raw materials in advanced therapy medicinal products: a CRO perspective
Alistair Michel, David Neville
25 March 2021
Innovator Insight

Regulatory FAQs & common concerns for cell & gene therapy raw & starting materials - ANIMATION
Kasey Kime, Jerrod Denham, Christopher Bravery
06 October 2020
Innovator Insight
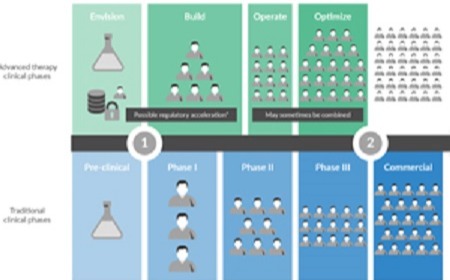
Commercial Lessons for Clinical Success
Simon Ellison, Heidi Hagen, Christophe Suchet
19 December 2018
Innovator Insight

Strategic & practical considerations in raw material selection & sourcing for cell therapies
David Wellis, Robert Tressler
10 April 2017
Innovator Insight
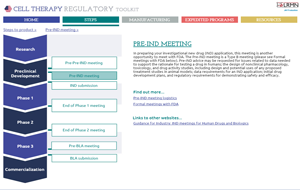
A US Cell Therapy Regulatory Toolkit
E Culme-Seymour, J Lawford-Davies, M Carpenter et al
14 September 2015
Innovator Insight